Abstract
Background
The advent of tyrosine kinase inhibitors (TKIs) has been dramatically improved outcome of Chronic Myeloid Leukemia (CML) patients. In TKI era, patients with CML has similar life expectancy compared to general population. Adult Comorbidity Evaluation 27 score (ACE-27) is a scoring system assessing the burden of co-morbidities reviewing 27 factors related to twelve organ systems, which has been extensively studied as a predictor of outcome in various medical settings. We hypothesized that ACE-27 has prognostic impact of newly diagnosed, chronic phase CML patients, treated TKI as a frontline therapy, in long-term outcome.
Methods
We reviewed patients with newly diagnosed chronic phase CML at the University of Texas MD Anderson Cancer Center from 2000 to 2014 who were enrolled in clinical trials. Patients who received TKIs (imatinib, dasatinib, nilotinib, or ponatinib) as a frontline therapy were included. Patient and disease characteristics, treatment details were collected prospectively, and further collected by retrospective chart review. ACE-27 score was calculated accordingly. We studied its association to treatment response, overall survival (OS) and event-free survival (EFS). Complete cytogenetic remission (CCyR) was defined as the absence of Philadelphia chromosome (Ph) by karyotyping. Major cytogenetic response (MCyR) is defined as less than 35% of metaphases with Ph chromosome. Major molecular response (MMR), molecular response (MR) 4.0, and MR4.5 are defined as BCR-ABL1 transcript ≤ 0.1%, ≤ 0.01%, and ≤ 0.0032% on the international scale, respectively.
Results
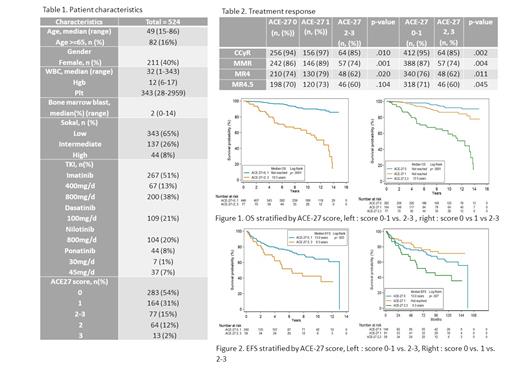
In a total of 524 patients, median age was 49 (range 15-86) with 16% of patients over age 65. Median follow-up time was 98 months. 267 (51%) patients were on imatinib, compared to 21% on dasatinib, 20% on nilotinib and 8% on Ponatinib. ACE-27 score was 0, 1, 2, and 3, in 284 (54%), 164 (31%), 64 (12%), and 13 (2%) patients, respectively. Detailed patient characteristics were summarized in Table 1. Patients with ACE-27 2-3 were less likely to achieve CCyR, MMR, MR4 or MR4.5 compared with those with ACE-27 0-1 (p=.010)(table 2). For ACE-27 2-3 patients, odds ratio (OR) for achieving CCyR, MMR, MR4 and MR4.5 compared to ACE-27 0-1 patients were 0.39 (p=.003), 0.43(p=.005), 0.52(p=.012), 0.60(p=.046), respectively. Time to response in each remission was similar between ACE-27 0-1 and ACE-27 2-3 groups. Overall survival was worse in ACE-27 2,3 patients with median survival at 12.5 years compared to not reached in patients with ACE-27 0 or 1, or 0 and 1 (p<.0001) (Figure 1). Hazard ratio(HR) for death in ACE-27 2-3 was 9.7(5.3-17.4, p<.001) compared to ACE-27 0. After adjustment with age ≥65, gender, sokal score, type of TKIs, still ACE-27 2,3 showed significant worse survival compared to ACE-27 0 or ACE-27 0, 1 at HR 7.18(95% CI 3.8-13.6, p<.001), HR 6.7(3.9-11.7, p<.001), respectively. When stratified by TKI types, ACE-27 2-3 remains statistically significant worse outcome in patients, though analysis on ponatinib and dasatinib was limited due to small number. ACE-27 2-3 patients also showed inferior EFS in ACE-27 2-3 with median EFS at 6.3 years(p=.007), but not well separated between patients with score 0 and 1(Figure 2 right, p= .262). HR for event in ACE-27 2-3 group was 1.81 (95% CI 1.09-2.99, p=.022) compared to ACE-27 0 group. When patients with ACE-27 0 and 1 are grouped together, survival curve is well-separated between ACE 27 0-1 and ACE 2-3 patients (Figure 2, p= .003).
Conclusion
ACE-27 score predicts outcome including overall survival and event-free survival well in chronic phase CML patients. Our study demonstrated that co-morbidities has significant impact on outcome of chronic phase CML on TKI treatment, and the potentials of ACE-27 as a part of prognostic instrument.
Kantarjian: AbbVie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Ascentage: Research Funding; BMS: Research Funding; Daiichi-Sankyo: Research Funding; Immunogen: Research Funding; Jazz: Research Funding; Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Aptitude Health: Honoraria; Astellas Health: Honoraria; Astra Zeneca: Honoraria; Ipsen Pharmaceuticals: Honoraria; KAHR Medical Ltd: Honoraria; NOVA Research: Honoraria; Precision Biosciences: Honoraria; Taiho Pharmaceutical Canada: Honoraria. Jabbour: Amgen, AbbVie, Spectrum, BMS, Takeda, Pfizer, Adaptive, Genentech: Research Funding. Issa: Kura Oncology: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Syndax Pharmaceuticals: Research Funding. Ravandi: Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding; Prelude: Research Funding; Jazz: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Taiho: Honoraria, Research Funding; Agios: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; AstraZeneca: Honoraria; Novartis: Honoraria; Amgen: Honoraria, Research Funding; Xencor: Honoraria, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. DiNardo: GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Foghorn: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; ImmuneOnc: Honoraria, Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Forma: Honoraria, Research Funding; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding; Takeda: Honoraria; Novartis: Honoraria; Agios/Servier: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Research Funding. Konopleva: Cellectis: Other: grant support; Forty Seven: Other: grant support, Research Funding; Calithera: Other: grant support, Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; Ascentage: Other: grant support, Research Funding; Agios: Other: grant support, Research Funding; AstraZeneca: Other: grant support, Research Funding; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; Ablynx: Other: grant support, Research Funding; KisoJi: Research Funding; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; Sanofi: Other: grant support, Research Funding; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; Stemline Therapeutics: Research Funding; Rafael Pharmaceuticals: Other: grant support, Research Funding. Jain: ADC Therapeutics: Honoraria, Research Funding; Cellectis: Honoraria, Research Funding; TG Therapeutics: Honoraria; Pharmacyclics: Research Funding; Servier: Honoraria, Research Funding; Fate Therapeutics: Research Funding; AbbVie: Honoraria, Research Funding; Precision Biosciences: Honoraria, Research Funding; Incyte: Research Funding; Adaptive Biotechnologies: Honoraria, Research Funding; Pfizer: Research Funding; Genentech: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Beigene: Honoraria; Janssen: Honoraria; Aprea Therapeutics: Research Funding; Bristol Myers Squibb: Honoraria, Research Funding. Pemmaraju: Celgene Corporation: Consultancy; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; Clearview Healthcare Partners: Consultancy; CareDx, Inc.: Consultancy; Pacylex Pharmaceuticals: Consultancy; LFB Biotechnologies: Consultancy; Sager Strong Foundation: Other; Bristol-Myers Squibb Co.: Consultancy; Incyte: Consultancy; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Springer Science + Business Media: Other; MustangBio: Consultancy, Other; Daiichi Sankyo, Inc.: Other, Research Funding; Protagonist Therapeutics, Inc.: Consultancy; Cellectis S.A. ADR: Other, Research Funding; Blueprint Medicines: Consultancy; Affymetrix: Consultancy, Research Funding; Roche Diagnostics: Consultancy; DAVA Oncology: Consultancy; ImmunoGen, Inc: Consultancy; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; Aptitude Health: Consultancy; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Samus: Other, Research Funding; Plexxicon: Other, Research Funding. Borthakur: ArgenX: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astex: Research Funding; University of Texas MD Anderson Cancer Center: Current Employment; Ryvu: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy; Protagonist: Consultancy. Kadia: Cure: Speakers Bureau; Dalichi Sankyo: Consultancy; Genentech: Consultancy, Other: Grant/research support; Jazz: Consultancy; AbbVie: Consultancy, Other: Grant/research support; Aglos: Consultancy; Pfizer: Consultancy, Other; Sanofi-Aventis: Consultancy; Ascentage: Other; Genfleet: Other; AstraZeneca: Other; Astellas: Other; BMS: Other: Grant/research support; Cellonkos: Other; Pulmotech: Other; Liberum: Consultancy; Novartis: Consultancy; Amgen: Other: Grant/research support. Cortes: Bio-Path Holdings, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb, Daiichi Sankyo, Jazz Pharmaceuticals, Astellas, Novartis, Pfizer, Takeda, BioPath Holdings, Incyte: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Sun Pharma: Consultancy, Research Funding. Sasaki: Pfizer: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal